
Using aseptic technique, inject 20 mL of Sterile Water for Injection, USP into the vial. Shake the vial until complete dissolution. The reconstituted solution is clear, colorless to pale brownish-yellow, and contains 0.05 mg/mL of trabectedin.

Obtain different resources on our solutions for microbiology in our Microbiology Solutions Resource Library.

Oct 30, 2017 · The CPID-CE constitutes part of the Notice of Compliance (NOC) package. The CPID-CE provides an accurate record of key quality information for the product proposed for marketing at the time the NOC is issued, and thereafter serves as an official reference document during the course of post-approval inspections and post-approval change evaluations as performed by Health Canada.

Biotech Kits; Electrophoresis. Carolina makes DNA gel electrophoresis easy when studying forensics or genetics. There's a simple set up with consistent results. Biotech Equipment & Labware. Outift your Biotechnology lab with Carolina Quality. A wide product selection—from gel chambers to power supplies, centrifuges and pipets.

Slide 3 Related Regulations and Guidelines •GMP regulations in EU and USA •ICH –Guidance documents are signed into regulations of member countries: e.g., Q7, Q8, Q9, Q3D

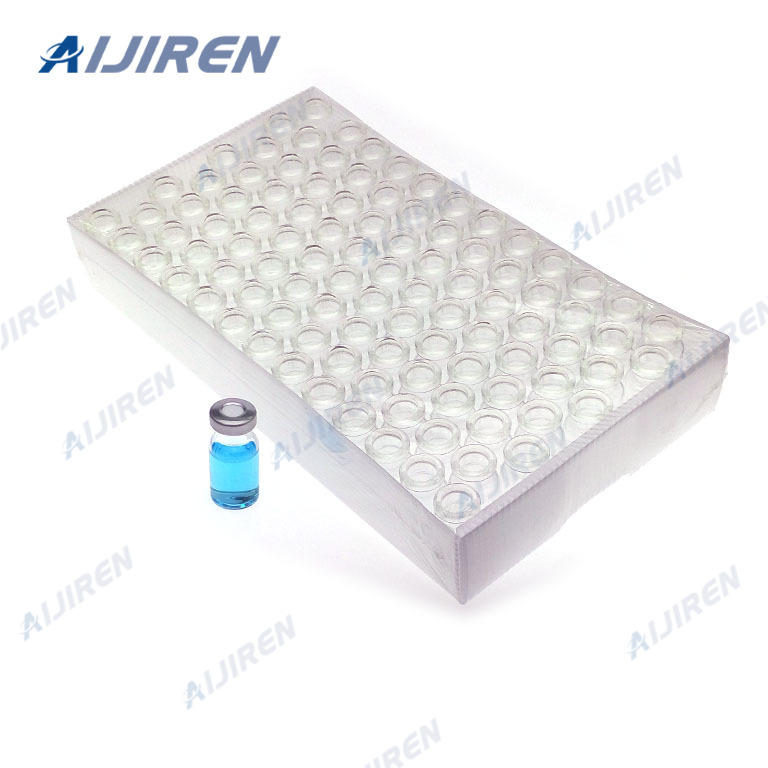
Aijiren clear glass vial inserts, polypropylene vial inserts and high recovery vials ensure you extract the maximum possible amount of your precious sample. Aijiren vial inserts are the perfect fit for HPLC and GC instrument platforms across a wide variety of applications, including pharmaceutical, environmental, energy and fuels, forensics, materials science, biopharmaceutical, proteomics, and metabolomics.

Antara™ (Polectron) 430 polymer is a white, thermoplastic, 38-41 percent solids, latex produced as a graft, emulsion copolymer of 30 percent polyvinylpyrrolidone and 70 percent styrene. With a glass transition temperature of approximately 100°C, it forms transparent, thermoplastic films that readily adhere to glass, plastics and metals.

Provide clear directions so the recipient can evaluate monitoring devices/indicators and determine if products should be accepted or rejected. 5.4 Receiving Receiving is an important role that may occur several times and in different locations along the distribution chain, including storage warehouses and the final distribution point.

The largest and most experienced supplier of horticulture, organics, hydroponics, and lighting supplies. We are a distributor for leading brand name suppliers such as Gavita, Eye Hortilux lamps, Grodan rockwool, General Hydroponics, Botanicare, Bloombastic, Atami, Hydro Dynamics, Roots Organics, Dutchmaster, Grotek, Earth Juice, Dyna Gro and many more

On the day of the collection, discard the first morning urine void, and begin the collection after this void. Collect all urine for the next 24 hours so that the morning urine void on the second day is the final collection. Measure and record this volume on the test request form and on the urine transport vial (see Pediatric Specimen Tubes below).
At Merck, we're following the science to tackle some of the world's greatest health threats. Get a glimpse of how we work to improve lives.

LCGC Certified Clear Glass 12 x 32 mm Screw Neck Vial, Waters Certified vials are tested for cleanliness by HPLC and held to tightest dimensional tolerances in the industry. SKU: 186000307C LCGC Certified Clear Glass 12 x 32 mm Screw Neck Vial, with Cap and Preslit PTFE/Silicone Septum, 2 mL Volume, 100/pk

Vial closure systems have undergone validation to ensure quality performance. See the most commonly used packaging configurations below. o Amber glass vials with Teflon-coated stoppers - typical packaging for USP RS o Clear, conical glass vials with Teflon-coated stoppers - for RS’s with small package size (e.g. 10-15 mg)

C - Liquid Nitrogen Vial Storage Capacity (back to chart) Cryogenic storage systems utilize racks compatible with 81-cell or 100-cell cryo-boxes to maximize sample capacity. Samples are generally stored in 1.5 ml or 2 ml cryo-tubes or twist-cap vials.

SCHOTT Vials DC (Delamination Controlled) Glass delamination resulting in barely visible glass flakes has led to recalls of numerous injectable drug products over the past few years. In order to prioritize patient safety, the FDA has reacted to this phenomenon by emphazising the importance of container/drug compatibility testing